Products and Services
Breast Cancer
Breast cancer patient samples can be cultured using 506386 Normal Breast Media and for transformed breast cancer cells use 506387 Transformed Breast Media (Ince BMI-T)
- The regular breast cancer media helps transition patient samples to stable cultures that retain the 95%+ of original sample.
- The transformed media helps establish breast cancer colonies that are more representative of the original breast cancer samples, reducing sample drift.
Ovarian Cancer
Culturing ovarian cancer patient samples is not easy. Some sources are more difficult than others, for example Ascites fluids are difficult to isolate and culture cancer cells from. The GLG INCE media to start with is the 506389 Primary Ovarian Cancer Establishment Media (Ince OCMI-e).
Once the cell lines are growing then use 506390 Primary Ovarian Cancer Maintenance Media (Ince OCMI-L).
- Establishment media helps retain the 95%+ RNA/DNA signature of the original sample
- Maintenance media helps culture cancer cells that histologically look more like the original sample
T-cell Leukemia
Leukemia samples can also be difficult to grow, the 506392 Primary T-cell Leukemia Media (Ince TLMI) helps establish a stable and reproducible cancer cell line.
- The T-cell Leukemia media helps establish cell lines that retain a majority of the original sample properties.
- Cancer cell lines can be expanded multiple times without compromising retention of cancer cell line signatures.
Products
| GLG Number | Product Name | Price | Size |
| 506387 | Transformed Breast Media (Ince BMI-T) (Liquid) | $666 |
500 ml |
| 506389 | Primary Ovarian Cancer Establishment Media (Ince OCMI-e) (Liquid) | $666 | 500 ml |
| 506392 | Primary T-Cell Leukemia Media (Ince TLMI) (Liquid) | $666 | 500 ml |
| 506390 | Primary Ovarian Cancer Maintenance Media (Ince OCMI-L) (Liquid) | $666 | 500 ml |
| 506386 | Normal Breast Media (Ince BMI-P) (Liquid) | $666 | 500 ml |
| 506388 | Normal or Transformed Ovary and Fallopian Tube Media (Ince FOMI) (Liquid) | $666 | 500 ml |
| 506391 | Primary Endometrioid and Mucinous Ovarian Cancer Media (Ince OCMI-Le) (Liquid) | $666 | 500 ml |
| 506388-01 | Normal or Transformed Ovary and Fallopian Tube Media w/o HCT (Ince FOMI) (Liquid) | $666 | 500 ml |
Customized Combinations of Media for Cancer Cells are Available by Special Order.
New Products:
GLG new products are:- Prostate Cancer Cell Culture Media
- Organoid Culture Media
GLG's newest media is for culturing organoids from any cancer tissue source or from immortalized or transformed cancer cell lines. The media is now available by special order: Contact Richard Gabriel at rgabriel@glgpharma.com
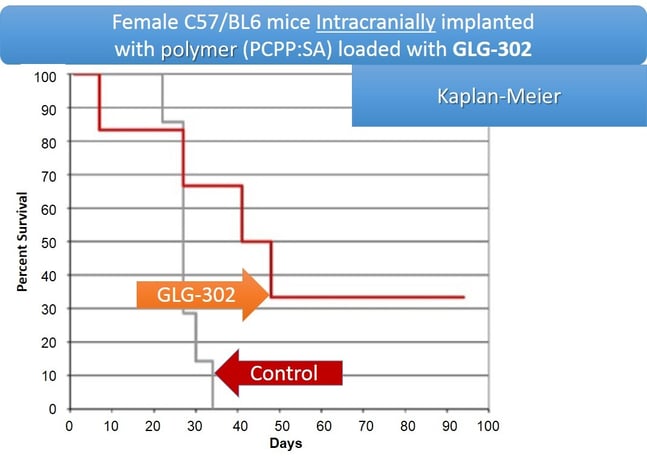
STAT3 Degrader Compounds
At GLG Pharma we believe the path to better cancer patient treatment starts and ends with the patient samples and how they are handled, processed, cancer cells are cultured and screened for drug response and other important profile characteristics. A part of that process is screening drug candidates for new therapeutic applications. Brain cancer is one of the hardest to treat cancers and having a compound imbedded in a slow release platform may be one way to treat brain cancer. Combining STAT3 degradation compounds with known intercranial drug treatment options can improve patient outcomes.
Meet Our Team

Richard Gabriel, BS, MBA
Co-Founder
Richard has been working in cancer since the mid 1980's. His background is chemistry and business, with broad experience in FDA/GMP drug discovery, development, and approval. His interests include cooking, rare books and ephemera, photography, hiking, and nature.

Hector J. Gomez, MD, PhD
Co-Founder
Hector has worked in the pharmaceutical and biotech industry resulting in 11approved drugs and working for the likes of Merck, Pharmacia, Vertex and others. Hector's drugs were the first to be approved by the US FDA using a nearly all South American patient populations. He enjoys golf, fine wines, swimming, and dancing. He has a very large extended family that he also very much enjoys.

