“Highlighting Chronic Lymphocytic Leukemia (CLL)”
The goal of every pharmaceutical and biotechnology scientist, physician and clinician is to save the patient’s life through an outright cure of the disease and if it can’t be saved then improve the quality of his/her life. Below are definitions of Rare Diseases, according to our friends at Evaluate Pharma[1]:
Evaluate Pharma Excerpt:
“The National Organization for Rare Disorders (NORD), which was instrumental in establishing the Act, currently estimates 30 million Americans suffer from 7,000 rare diseases. Prior to the 1983 Act, 38 orphan drugs were approved. To date, 468 indication designations covering 373 drugs have been approved. The success of the original Orphan Drug Act in the US led to it being adopted in other key markets, most notably in Japan in 1993 and in the European Union in 2000. Rare Disease Patient Populations are Defined in Law as:
- USA: <200,000 patients (<6.37 in 10,000, based on US population of 314m)
- EU: <5 in 10,000 (<250,000 patients, based on EU population of 506m)
- Japan: <50,000 patients (<4 in 10,000 based on Japan population of 128m)
Financial Incentives by Law Include: Market Exclusivity
- USA: 7 Years of marketing exclusivity from approval. Note: Majority of orphan drugs have a compound patent beyond 7 years. The market exclusivity blocks ‘same drug’ recombinant products, e.g. Fabrazyme (Genzyme, now Sanofi) vs. Replagal (Transkaryotic, now Shire). ‘Same drug’ exclusion can be overturned if clinically superior (mix of efficacy/ side effects), e.g. Rebif overturned Avonex’s orphan drug exclusivity (7 MAR 2002)
- EU: 10 Years of marketing exclusivity from approval.
Reduced R&D Costs:
- USA: 50% Tax Credit on R&D Cost
- USA: R&D Grants for Phase I to Phase III Clinical Trials ($30m for each of fiscal years 2008-12)
- USA: User fees waived (FFDCA Section 526: Company WW Revenues <$50m)
Methodology on Classifying an Orphan Drug:
"We, (Evaluate Pharma) have identified all products that have orphan drug designations filed in the US, EU or Japan. These are available as part of the core EvaluatePharma service. To further enhance analysis, we have defined a clean ‘Orphan’ sub-set of products following a number of criteria including:
- First indication approved is for an orphan condition.
- Products expected to generate more than 25% of sales from their orphan indications.
This has led to the exclusion of drugs such as Avastin, Enbrel, Herceptin, Humira and Remicade, all of which have orphan designations for indications contributing less than 25% of sales.
- Trial sizes, with smaller Phase III trials suggesting orphan status.
- Drug pricing, higher prices were taken as an indicator of orphan status.
- All sales analysis in the report is based on this clean ‘Orphan’ sub-set of products.” End of Evaluate Pharma excerpt.
Chronic Lymphocytic Leukemia (CLL) is considered a ‘rare disease’. A great source of information on CLL[2] is the Leukemia & Lymphoma Society[3] which has over the years poured over $1 billion into research for Leukemia. The information found in the PDF download (see reference) is excellent and is a foundation for understanding this disease and other leukemia’s as well.
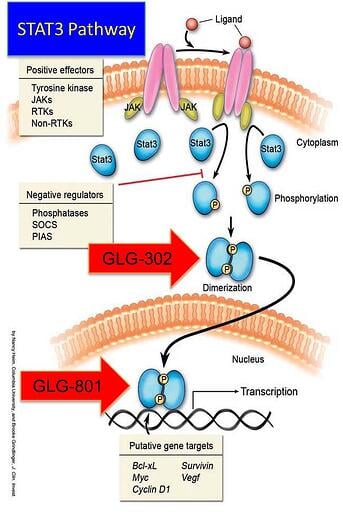
Some rare diseases have identified mechanisms of action that lay across the biological human horizon of diseases but aren’t as highly expressed or manifest themselves as a chronic lethal disease. One of the mechanisms sometimes associated with proliferative diseases that includes rare forms of cancer, is an abnormality in a major signaling pathway located downstream where many other pathways convey extracellular signals into the nucleus. This is the case of the Signal Transduction and Activators of Transcription (STAT) and in particular, STAT3.
At GLG Pharma we have focused on the STAT3 signaling pathway and its uncontrolled hyperactivity. Activation of STAT3 can be blocked at three different sites:
- Phosphorylation
- Dimerization
- DNA Binding
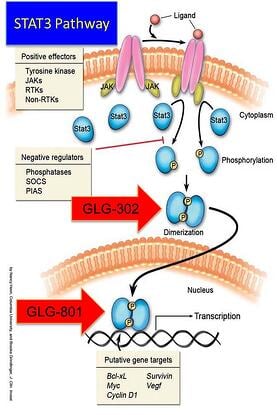
Three drugs are in the development pipeline. The first with the shortest path to the market is GLG-801. This is a repurposed drug and under US FDA rules for 505(b)(2) could be fast tracked for various indications and clinical trials might be initiated, as soon as funds become available, in patients with rare diseases such as Chronic Lymphocytic Leukemia (CLL) and Gastro-intestinal Stromal Tumors (GIST). GLG-302, a new chemical entity (NCE) follows in the development cycle and is expected to be in the clinic in about 8 months from funding. GLG-202 is another NCE that will follow in the development cycle. GLG-801 inhibits DNA binding and both GLG-302 and GLG-202 inhibit dimerization of the STAT3 molecule, preventing its penetration of the nuclear membrane and the initiation of the transcription process and the continuation of the uncontrolled proliferation process.
Want to know more about GLG Pharma and Poliwogg? Raising awareness and helping us fight cancer! Then click on the Poliwogg picture and it will take you to the Poliwogg accredited investor site!
Want to find out more about what compounds are in the clinic for CLL? Then click the button. Once you have signed up, we will email you the PDF document that provides you with compound structures and data on the activity of the compounds as well as their site of action on the leukemia cells!