FOR IMMEDIATE RELEASE
GLG Pharma is participating along with Rare Disease Advocates Commemorate Worldwide Awareness Day at the Florida State House
Tallahassee, Florida, February 19, 2015----- Join rare disease patients, caregivers and other health care advocates as they share their stories on February 19, 2015, to observe Rare Disease Day in Florida. Rare Disease Day occurs each year on the last day of February, and on this day, millions of patients and their families will share their stories to focus a spotlight on rare diseases as a global public health concern. The day is observed in more than 70 nations.
Patient advocates have joined with the National Organization for Rare Disorders (NORD), the national sponsor of the day in the US, to organize this special event for legislators, legislative staff, the public and the media. The event will be held in the State Capitol in the entry level of the Rotunda, from 9:00 am –11:00 am.
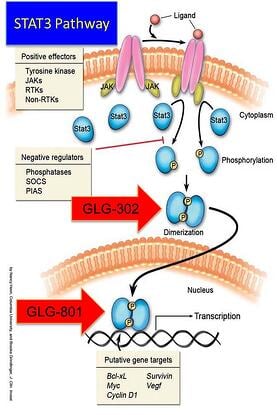
During this event attendees will have the opportunity to meet/ hear from Florida Rep. Mike La Rosa District 42, David Dittman – Shriners Hospital, Tampa, Bella’s Fight, Felecia Peete adoptive mother of 3 boys who are affected with ALD, and Shannon Brown-Brinson – United For ALD, Inc. The topic we will be discussing will be common challenges that the rare disease community faces pertaining to care, diagnosis, and awareness. GLG Pharma will have a table, featuring its STAT3 inhibitors that may be used to combat rare diseases. GLG have identified over 45 rare diseases where the mechanism of STAT3 is turned on leading to excessive cell proliferation. Normally, STAT3 is only turned on when a cell in body divides. In cancer and proliferative diseases or diseases where cell proliferation is a product of the disease, STAT3 can be turned on up to 100% of the time.
The purpose of this event is to raise awareness at the state level for the 1 in 10 individuals living with a rare disease and the challenges they face. Many important decisions related to rare diseases are made at the state level, and the implementation of the Affordable Care Act has highlighted the increasingly important role of state policies and programs in assuring that the healthcare needs of the American public are addressed.
Issues of importance to the rare disease community that may be decided at the state level including newborn screening, support services for families coping with complex medical needs, an environment that promotes innovative medical research and product development, and insurance practices that assure patient access to medically necessary therapies.
A rare disease is one that affects fewer than 200,000 Americans. There are nearly 7,000 such diseases affecting nearly 30 million Americans, according to the National Institutes of Health (NIH). Two-thirds of those affected by rare diseases are children, and the diseases tend to be serious and lifelong. Even so, most rare diseases have no approved treatment, and many are not even being studied by medical researchers. Often, research on rare diseases is funded by the families and friends of patients or by patient organizations.
Participating Organizations Include: NORD, United For ALD, Inc., and Shriners Hospital in Tampa
Rare Disease Day was launched in Europe in 2008 by EURORDIS, the organization representing rare disease patients in Europe. It is now observed in more than 65 nations, and is sponsored in the U.S. by NORD.
For more information about Rare Disease Day in the U.S., go to www.rarediseaseday.us. For information about global activities, go to www.rarediseaseday.org).
For more information about GLG Pharma visit our website at http://www.glgpharma.com
For more information if you are an accredited investor interested in helping GLG Pharma focus on rare diseases then visit http://www.poliwogg.com and register. It’s safe, secure and confidential. There is a better way to fund Biotech!